Cloud Storage vs. On-Premises Servers for Surgical Video
![]() Theator
on
August 6, 2024
Theator
on
August 6, 2024
3 Critical Considerations for Storing Surgical Video Data
In the digital age, hospitals increasingly rely on data to enhance patient care and streamline operations. A critical decision for hospitals, especially when dealing with sensitive surgical data, is choosing between on-premise storage and cloud storage.
Each option has its own set of advantages and disadvantages, particularly in terms of security, accessibility, and cost. Which option is a better fit for health systems?
Spoiler alert: It’s the cloud! (At least in our opinion). But want to find out why? Keep on reading!

1. Security
Security is paramount when it comes to storing surgical data. Hospitals must ensure that patient information is protected from breaches and unauthorized access. On-premise storage offers a sense of control, as data is stored within the hospital’s own infrastructure. However, this control comes with the responsibility of maintaining robust security measures, including physical security, encryption, and regular updates to guard against cyber threats.
Cloud storage, on the other hand, leverages the expertise of specialized security teams employed by the cloud service providers. These providers invest heavily in security technologies and practices, often exceeding what individual hospitals can implement. Data stored in the cloud is encrypted both in transit and at rest, and providers comply with stringent regulations and standards, such as HIPAA, to ensure the protection of sensitive health information.
While some may have concerns about the perceived vulnerability of storing data off-site, cloud providers offer advanced security measures, such as multi-factor authentication, intrusion detection systems, and continuous monitoring. The distributed nature of cloud infrastructure also adds a layer of redundancy, protecting data against local hardware failures or disasters.
2. Accessibility
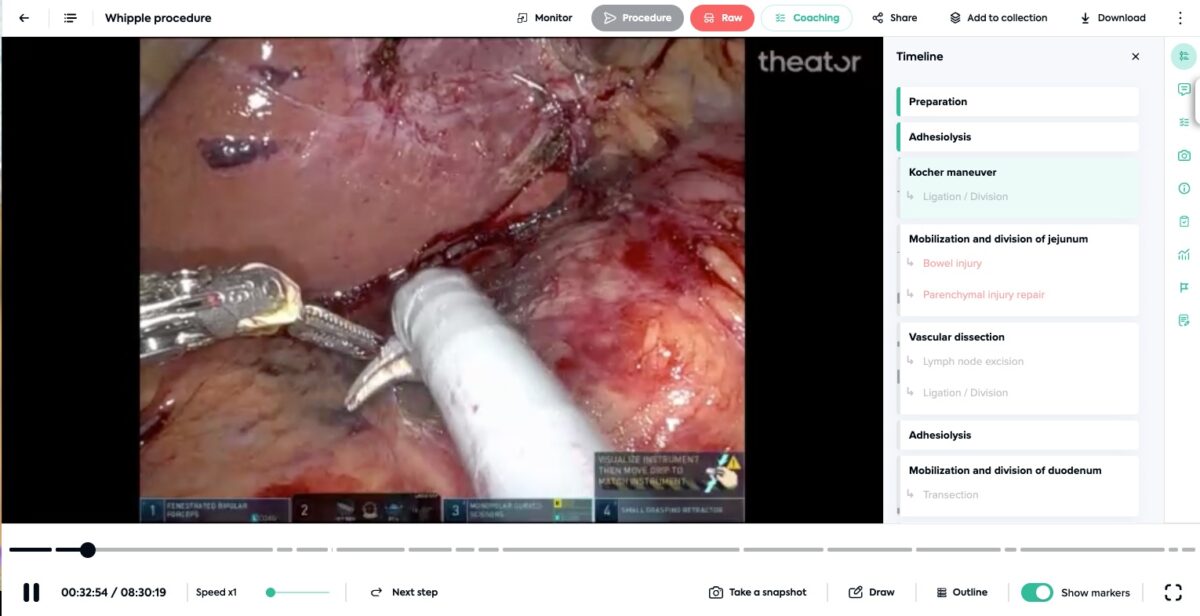
Email, texts, music and more are always accessible via our phones on-demand. Your surgical videos can be accessible, too, but it is a lot more likely that is true if they are stored on the cloud.
On-premise storage systems typically require users to be on-site or use a VPN to access data remotely, which can be cumbersome and slow. This limited accessibility can hinder the efficiency of surgical teams and the ability to make timely, informed decisions.
Cloud storage offers unparalleled accessibility. Data can be accessed from anywhere with an internet connection, using a variety of devices, including smartphones, tablets, and laptops. Need to check on something but you are already home from the hospital? No problem. Real-time access to data can enhance coordination and improve patient outcomes.
3. Cost: On-Premise vs. Cloud
One of the most significant considerations in the decision-making process is cost. On-premise storage requires substantial upfront investments in hardware, software, and infrastructure. Hospitals need to purchase servers, employ IT staff to manage the system, and regularly update the technology to keep up with advancements. Budget also needs to be set aside for maintenance and also the energy costs for running and cooling the servers.
In contrast, cloud storage operates on a subscription model, where hospitals pay for the storage capacity they use. This model eliminates both the need for large initial capital expenditures and ongoing maintenance costs. Cloud providers like Amazon Web Services, Google Cloud, and Microsoft Azure offer scalable solutions that adjust to a hospital’s changing storage needs. Moreover, cloud storage can be more cost-effective over time, as the costs of infrastructure, security, and updates are spread across many users and managed by the cloud provider.
Why Theator Chooses Cloud Storage
Simple – it’s secure, always accessible, and comes at a lower cost.
How much can storing videos on the cloud save a hospital compared to on-prem? Fill out the form below and we’ll email you your custom savings. If you’re unsure of the exact numbers, an estimate will be sufficient to give you an idea of potential savings.